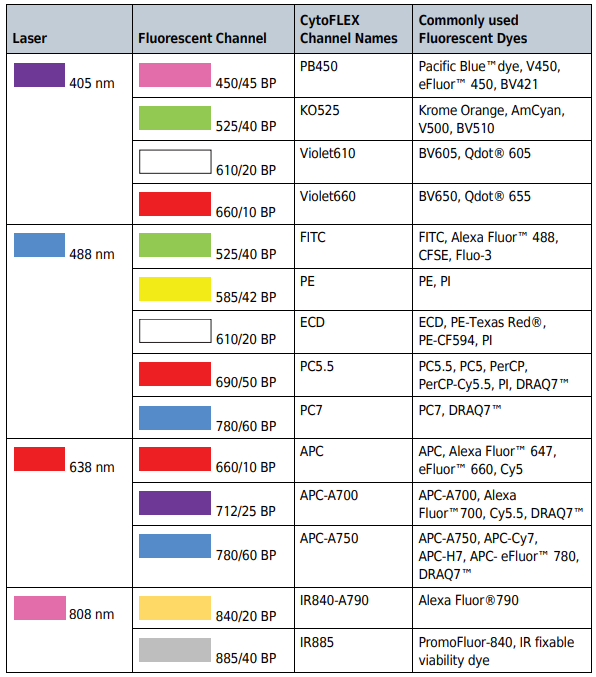
ResearchDx provides flow cytometry services utilizing the Beckman Coulter CytoFLEX S flow cytometer. Our CytoFLEX S’s are equipped with 4 lasers (405 nm, 488 nm, 638 nm, and 808 nm) that can perform multiplex testing with up to 13 biomarkers.
Cell-profiling / Immunophenotyping Immunophenotyping is the analysis of heterogeneous populations of cells with the purpose of identifying the presence and proportions of varying populations of interest. A given cell population can be identified and quantified by evaluating the unique immunological cell markers present using one or several labeled antibodies. Many cell markers are CD (cluster of differentiation) markers and are commonly used for the detection of specific immune cell populations and subpopulations. Some examples are listed below:
Receptor Occupancy Assays are designed to quantify the binding of antibody-based therapeutics (monoclonal antibody or an antibody conjugated with a therapeutic component) to their targets on the cell surface and are frequently used to generate PD (pharmacodynamic) biomarker data which can be coupled with PK (pharmacokinetic) profiles to model PK/PD relationships in nonclinical and clinical biopharmaceuticals studies.
Measures unbound receptors on a cell by using an unlabeled anti-receptor antibody and a competing therapeutic antibody/drug-conjugated antibody. The binding of the unlabeled antibody is measured by using a labeled secondary antibody to calculate unoccupied receptors. This is a critical measurement when assessing the cell specificity and therapeutic dose of an antibody.
Measures a competing therapeutic antibody binding to its receptor site and a non-competing alternate antibody that binds to a different site on the same receptor. This measures total receptor expression on cells and provides insight into the level of receptor internalization from the therapeutic antibody binding.
Rare events analysis has growing importance for the diagnosis and monitoring of immunological and hematological disorders. Rare circulating tumor cells (CTC) and rare circulating endothelial cells (CEC) are useful as disease indicators. Detection and function of rare cells could give relevant information about the status and stage of disease.
Functional cell-based assays are a key component in drug discovery and development, such as determining the efficacy and toxicity of drug compounds. Functional cell-based assays enable high-throughput compound screening to measure the functional behavior of the cell of interest in response to the compound. Some examples are listed below:
Intracellular cytokine staining is used for the analysis of intracellular proteins that are expressed by specific cell types and provides information concerning cellular function and signaling responses. Antibodies specific to cell surface markers can be combined with markers for intracellular cytokine production, proliferation, and protein phosphorylation to determine the cellular subsets’ response to various types of treatment.
A phosphoflow assay is used to examine the phosphorylation status of specific proteins that govern cellular responses through signaling pathways; the phosphorylation and dephosphorylation of regulatory signaling proteins can be used to evaluate the underlying mechanisms in therapeutic intervention, such as drug sensitivity and resistance.
CAR (Chimeric Antigen Receptor) cells are comprised of a recombinant receptor construct expressed in T cells to target cells expressing specific antigens; CAR-T cells destroy tumor cells in certain B-cell cancers with additional studies in progress for solid tumors. However, CAR T-cells induce toxicities, such as CRS (Cytokine Release Syndrome) from T-cell activation or from on-target, off-tumor effects; therefore, circulating CAR T-cells into peripheral blood samples should be characterized as a monitoring strategy.