Next Generation Sequencing Services
Highlights of Our Targeted Panel, Exome, and Genome Sequencing Service
Here are a few examples of what we offer:
Library Preparation
|
Sequencing Modalities
|
|
Capture Technologies
|
Cancer Sequencing Panels
|
Roche Avenio Validated Panels
The Targeted Panel:This allows for the analysis of 17 oncology related genes, including those published in the National Comprehensive Cancer Network (NCCN) guidelines. The Expanded Panel:The expanded panel includes the 17 NCCN guideline related genes as well as 60 additional biomarkers that are currently being analyzed in oncology clinical trials to determine their utility The Surveillance Panel:True to its name the surveillance panel has been optimized with the guideline genes as well as 180 additional genes to allow for longitudinal analysis that monitors tumor burden (TMB) and minimal residual disease (MRD). |
 |
Illumina TSO500
The TruSight Oncology (TSO) 500 Panel:The TSO panel is designed for the SNV, Indel, CNV and fusion analysis of 523 oncology related genes making it a great biomarker discovery tool. It also analyzes Tumor Mutational Burden (TMB) and Microsatellite Instability (MSI). The TruSight Tumor 170 PanelComprehensive next-generation sequencing (NGS) assay that targets DNA and RNA variants from the same FFPE sample.
|
 |
CUSTOM panel development
|
ResearchDx has the experience and expertise to develop and validate your custom NGS assay. ResearchDx can optimally design Small (<20 targets) medium (20-200 targets) or Large (200-22000 targets) target panels using a range of technologies including both hybrid capture and amplicon. Need a custom panel, please Schedule a consultation to discuss your Next Generation Sequencing Service needs. |
 |
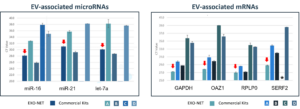
EXO-NET® Pan-Exosome RNA and micro-RNA Analysis
|
EXO-NET® Pan-Exosome Capture is an immunoaffinity magnetic bead-based isolation system, for exosome isolation from plasma, serum, urine, saliva and cell-conditioned medium. ResearchDx has validated Exosome solutions including:
EXO-NET is the highest-efficiency exosome isolation solution available ResearchDx is a certified provider of EXO-NET exosome sequencing services. Please contact us to learn more. |
 |
Flexible Bioinformatics Analysis and Data Storage
|
 |
Schedule a consultation to discuss your Next Generation Sequencing Service needs.
Our CAP/CLIA-certified lab, PacificDx offers state-of-the-art facilities and expertise to meet your immediate clinical and pre-clinical needs for high-quality clinical and biopharmaceutical testing services.