Featuring Industry Leading Panels and Custom Solutions
Our Validated Panels
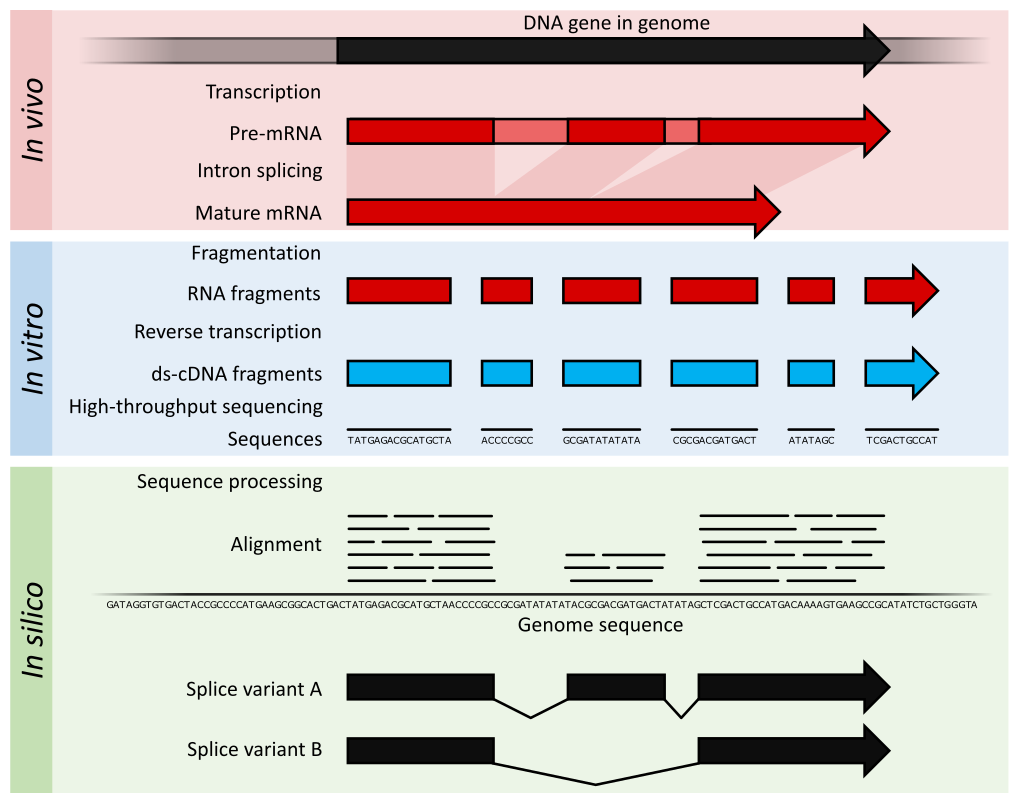
miRNA-seq and RNA-seq Analysis
- qPCR Expression Analysis
- miRNA NGS expression analysis
- Total RNA NGS expression analysis
What is a Liquid Biopsy
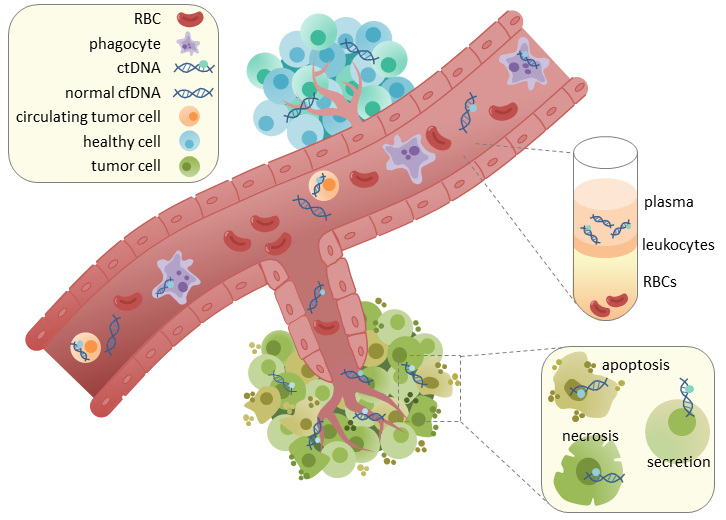
A liquid biopsy refers to capturing and analyzing circulating nucleic acids and proteins in biological fluids. These biological molecules may be free floating in the fluid, for example, cf-DNA, or may be encapsulated in subcellular structures, including exosomes, and circulating tumor cells in the context of cancer. Liquid biopsy tests are often designed for early cancer detection and treatment response monitoring when tumor tissue is insufficient or unavailable, or the patient needs a non-invasive option.
ResearchDx offers comprehensive cancer screening support – including bioinformatics for clinical trials, retrospective studies, or method comparisons. Our team of experts can run all of the panels on the list below or any custom panel.
Don’t see what you need. We have experience in whole genome/exome/transcriptome sequencing, RNA-Seq, minimal residual disease (MRD) monitoring, and custom panel development.