
ResearchDx specializes in providing a wide range of Regulatory Affairs, Clinical Affairs and Quality Assurance services to early-stage and established In Vitro Diagnostic (IVD) and Medical Device companies.
General Regulatory Filings and Consulting
We offer regulatory consulting and assistance for FDA & IVD compliance. Our team of scientists, biostatisticians, medical writers, project managers, clinical managers, and regulatory strategy experts navigate these pathways daily. Supported by an in-house team and an integrated CLIA, GCP, and GLP-compliant clinical laboratory, data management and regulatory submission becomes a simplified process for your medical products. Please schedule a consultation with our research team to discuss your specific needs. From concept to commercialization or for discrete projects in between, we provide all necessary diagnostic and regulatory support to complete your project promptly.
- Consulting services ensuring compliance with CFR 21 Part 820.
- External compliance and pre-inspection audits
- Documentation development and review
- Comprehensive IVD development end-to-end implementation
- Regulatory Strategy and Pathway Determination
- Facilitation of activities with domestic and international regulatory authorities
- Pre-submissions, 513(g) IVD PRE-IDE, PMA, and 510(k) filings
- Technical File and Design Dossier preparation and maintenance
- Product Labeling
- Facility Registration
- Warning Letter Mitigation
- Compliance auditing and consulting
- Software documentation
- Third-party review by Accredited Persons (AP)
- On-site consulting for product development projects
FDA Device
Quick Reference Guide
Design Control Quick Reference
Quality System Quick Reference
A 513(g) is a request for classification information from the FDA. The purpose of the submission is to ask the FDA what product classification and regulatory pathway would be most appropriate for your device.
The Q-Submission Program consists of 4 separate submission types; Pre-Sub requests, Submission issue requests, Study Risk Determinations, and Informational meetings
A Pre-Sub includes a formal written request from a submitter for feedback from FDA that is provided in the form of a formal written response or, if the submitter chooses, formal written feedback followed by a meeting in which any additional feedback or clarifications are documented in meeting minutes. A Pre-Sub provides the opportunity for a submitter to obtain FDA feedback prior to any intended premarket submission (i.e., IDE, PMA, De Novo request, 510(k), IND), Accessory Classification Request.
A SIR is a request for FDA feedback via written feedback or a meeting on a proposed approach to address issues conveyed in a marketing submission hold letter, a CW hold letter, an IDE Letter, or an IND Clinical Hold letter
Study Risk Determination is a request for FDA determination for whether a planned medical device clinical study is significant risk (SR), non-significant risk (NSR), or exempt from IDE regulations as defined by the IDE regulations (21 CFR part 812). For studies that are not exempt, sponsors are responsible for making the initial risk determination (SR or NSR) and presenting it to the Institutional Review Board (IRB).
An Informational Meeting is a request to share information with FDA without the expectation of feedback. This information sharing can be helpful in providing an overview of ongoing device development (particularly when there are multiple submissions planned within the next 6-12 months) and familiarizing the FDA review team about new device(s) with significant differences in technology from currently available devices. While FDA staff may ask clarifying questions during an informational meeting, they will generally be listening during the meeting and not prepared to provide any feedback.
There are 3 classes of medical devices: Class I,II, and III. The regulatory controls for each device class include: Class I (low to moderate risk) which require general controls. Class II (moderate to high risk): which require general controls and/or special controls and/or 510(k) clearance. Class III (high risk): which require general controls and Premarket Approval (PMA)
Most Class I devices are exempt from FDA requirements for Premarket Notification (510(k)) and Premarket Approval (PMA).
All medical devices including Class I require General Controls which speak to adulteration, misbranding, device registration, records and good manufacturing practices.
Medical device manufacturers who fall into Class I are still required to implement a quality management system and follow standards to ensure a quality product.
The FDA defines Class II devices as “devices for which general controls are insufficient to provide reasonable assurance of the safety and effectiveness of the device.”
Practically, Class II medical devices are those devices that have a moderate to high risk to the patient and/or user.
Class II devices required general controls and may require additional special controls.
Controls vary depending on the device, may include:
- Device performance
- Post-market surveillance
- Patient registries
- Special labeling requirements
- Premarket data requirements
- Guidelines
Most Class II devices require a premarket notification 510(k) submission. A 510(k) submission involves performing analytical showing ‘substantial equivalence’ to the existing marketed device (predicate).
The FDA defines class III devices as performing functions which “usually sustain or support life, are implanted or present a potential unreasonable risk of illness or injury.”
Class III devices are the highest risk category. subject to all General Controls and the FDA’s Premarket Approval (PMA) process. A PMA submission typically requires both analytical and clinical studies sufficient to provide the safety and effectiveness of the device design and operation.
The provisions of General Controls address
- Adulteration;
- Misbranding;
- Device registration and listing;
- Premarket notification;
- Banned devices;
- Notification and repair, replacement, and refund;
- Records and reports;
- Restricted devices; and
- Good Manufacturing Practices.
In practice, general controls are required for all three classes of medical device.
Special controls are regulatory requirements for class II devices. FDA classifies into class II devices for which general controls alone are insufficient to provide reasonable assurance of the safety and effectiveness of the device, and for which there is sufficient information to establish special controls to provide such assurance.
Special controls are usually device-specific and include:
- Performance standards
- Post market surveillance
- Patient registries
- Special labeling requirements
- Premarket data requirements
- Guidelines
General Special controls are required for some but not all Class II devices.
Premarket approval (PMA) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Class III devices support or sustain human life and are of substantial importance in preventing impairment of human health or presenting a potential, unreasonable risk of illness or injury.
A 510(k) Is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device (“Predicate” Section 513(i)(1)(A) FD&C Act) that is not subject to premarket approval.
A DeNovo request provides a marketing pathway to classify novel medical devices for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device. De Novo classification is a risk-based classification process. Devices that are classified into class I or class II through a De Novo classification request (De Novo request) may be marketed and used as predicates for future premarket notification 510(k) submissions, when applicable.
Medical Device & IVD Regulatory Consulting Services
Regulatory Strategic Planning and Due Diligence
- Craft targeted strategies for regulatory approval of healthcare products, including medical devices and in vitro diagnostics.
- Risk Assessment and Due Diligence evaluation
- Assist with Device Registrations
FDA Interactions and Meeting Facilitations
- Provide client representation during FDA engagements
- Assist client in strategic preparations for FDA meetings
Device Listing and Author Regulatory Submissions
- •Assist in preparing regulatory submissions for medical devices/IVDs such as 510(k)s, PMAs, IDEs, de novo requests, Pre-Submissions and 513(g)s.
- Refine testing protocols for bench, animal, and clinical trials, and interpret results to strengthen submission outcomes.
- Device Listing and Establishment Registration
In Vitro and Companion Diagnostic Services
- Offer expert technical guidance on the development and regulatory strategy of IVDs including companion diagnostics and personalized medicine.
- Design and management of analytical and clinical validation studies
Quality System and MD Compliance
- Offer in-house training sessions on FDA regulatory issues and updates on new policy
- DHF, DMR, DHR Creation and Review
Global Regulatory Submission
- EU MDR, IVDR and other international regulatory submission
- Performance Evaluation Plan, PMCF, PSUR, GSPR, Post Market Surveillance, Manufacturers and Device Registration
Device Classification
The Food and Drug Administration (FDA) has established classifications for approximately 1,700 different generic types of devices and grouped them into 16 medical specialties referred to as panels. Each of these generic types of devices is assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. The three classes and the requirements which apply to them are:
Device Class and Regulatory Controls
1. Class I General Controls
- With Exemptions
- Without Exemptions
2. Class II General Controls and Special Controls
- With Exemptions
- Without Exemptions
3. Class III General Controls and Premarket Approval
ResearchDx can provide expert assistance in classifying your medical device. Please do not hesitate to contact us.
FDA Meeting Preparation
FDA meetings introduce your product to the US regulatory authorities and are critical to the success of your diagnostic.
RDx has expertise to assist companies with:
- Initial client interactions with FDA
- Assist with preparations for FDA meetings such as Q-Sub pre-submission meetings
- Develop presentations for FDA meetings
- Prepare clients for Advisory Panel meetings
- Advise on regulatory options and potential pathways
FDA Submission Consulting Services
RDx develops and reviews a variety of regulatory submissions for FDA. From the beginning discussions with FDA to the marketing submission, RDx can develop, write, and review all FDA submissions. Often starting with the pre-submission document and continuing into the IDE or IND, along with all the reports and supplements, and ending with the marketing submission. Our experience includes:
• Pre-Submissions
• 510(k)
• IDE
• PMA
• IND
• BLA
• De Novo
• Breakthrough Designations
• Reports for PMAs, IDEs
• Materials for meetings with FDA including briefing packages, presentations, and executive summaries
The RDx approach ensures our integrated services provide a full scope assessment that ensures flawless interactions with regulatory agencies, from initial strategy, meetings and Submissions.
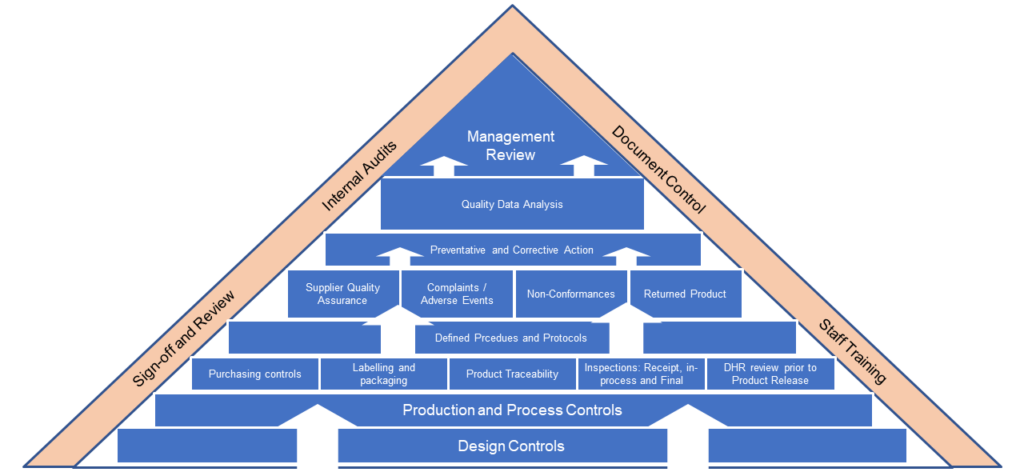
Quality System Support and Auditing
ResearchDx can provides expert consulting services to ensure your QMS system meets all FDA Quality Standards. Our services include:
- ISO 13485 Manufacturing
- ISO 15189 LAboratory
- ISO 14971 Risk Management
- US FDA 21 CFR Part 820
- EU MDR / AIMDR / IVDR
- Health Canada CMDR
- Australian TGA
- Japan MHLW Ordinance 169
Nonclinical Program Support
Reduce short- and long-term costs through a robust nonclinical program with support in methodology and strategy, study design, development, and accountability for program execution.
Clinical Program Support
Move your product efficiently and successfully through the clinic using a customized clinical design solution.
Commercial
Maximize the potential of your program throughout development and the commercial lifecycle with strategic insights that position your product for success.
