
Broad Custom Assay Development Capabilities
ResearchDx offers state-of-the-art custom and molecular assay development, from concept and biomarker discovery, through clinical validation and regulatory approval to final kit manufacturing. We can step in at any stage in the process to support your project needs. The ResearchDx team is highly skilled in all project phases associated with regulated and non-regulated environments in both the United States and abroad, supporting diagnostic development partnerships worldwide. Offering a diverse array of platforms and technologies, we can support all development and testing needs, molecular and non-molecular. An example list of platforms and technologies we support is found here. We continue to stay current with the latest advances in new technology and instrumentation.
Let us Build Your Next Custom NGS Panel
- Tumor profiling
- Cancer liquid biopsy
- Pharmacogenomics
- Neurology
- Cardiology
- Infectious disease
Or Provide NGS Solutions for Virtually Any Project
- Amplicon Sequencing
- RNA Sequencing
- Targeted Sequencing
- Viral Genome Sequencing
- Epigenomics
- Exosome Analysis
Let us Design your Next PCR, qPCR, or ddPCR Assay
- Gene expression
- Copy number variation
- Absolute copy number variation (CNV)
- Confirmatory testing
- SNP genotyping and allelic discrimination
- Methylation analysis (CpG)
- Pathogen detection and quality control
- Rare mutation and allelic discrimination
- Low-abundance transcript detection
- Viral titer (copies/mL)
- Vector copy number (copies/genome)
- Circulating tumor DNA (ctDNA) and cell-free DNA (cfDNA) detection in liquid biopsy
We also Build Preclinical In-Vitro Assays
- Flow Cytometry
- ELISA
- Cell proliferation
- Cell Viability
- Toxicity studies
- Immuno Assays
- Functional Assays
- PK Testing
Generate Control Material
- 100+ characterized control cell lines for oncology applications
- FFPE control cell block preparation
- Control DNA/RNA preparation
And Design, Validate and Implement Laboratory-Developed Tests (LDT’s)
Laboratory-Developed Tests (LDTs) have garnered significant attention in recent years over questions relating to FDA oversight, clinical and analytical validity, and compliance with certain medical device regulations. Though having similar usage as an FDA-approved IVD test, an LDT is created and validated for use by a single clinical laboratory. LDTs continue to be important in the advancement of personalized medicine which has highlighted the importance of accuracy, clinical and analytical validity. To this end, regulatory authorities are increasingly requesting such studies under the more stringent CLIA regulations. At ResearchDx, we design and perform analytical and clinical validations for custom assays through our PacificDx clinical laboratory. Our CAP/CLIA certified lab, PacificDx offers state-of-the-art facilities and expertise to meet your immediate clinical and pre-clinical needs for high-quality clinical and biopharmaceutical testing services.
Fit For Purpose Development
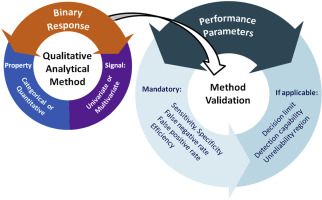
What Level of Validation Do I require
Research Assays:
Research grade tests are usually utilized in universities or “biomarker discovery” settings. There are no specific recommendations for Research level validation. Still, at ResearchDx, all validations include an assessment of accuracy, precision, detection limits and reporatble range sufficient to demonstrate the assay is suitable for the intended use.
GLP Validation:
The objective of assay validation is to demonstrate the assay is suitable for the intended use. The regulations do not outline the requirements for validation. Still, at a minimum, a GLP validation will include documentation of accuracy, precision, and limits of detection, plus any additional studies required to demonstrate “Fit for purpose”. GLP-level assays where the data is intended for FDA submission will require additional studies.
CLIA Assay Validation:
CLIA assays are typically utilized in a routine clinical setting or for clinical studies where the testing has the potential to impact patient care. CLIA clearly defines analytical validation requirements regarding accuracy, repeatability, reproducibility, detection and quantitation limits, reportable range, and interfering substances. CLIA accrediting agencies (Including CAP and A2LA) have specific requirements that may require additional studies.
ISO-13485 Assay validation:
ISO 13485:2016 specifies the requirements for a medical device manufacturer’s quality management system (QMS). The standard outlines the QMS requirements to prove that the manufacturer can produce safe and effective medical devices that meet user needs and comply with all applicable regulations.
510(k) and PMA Validated Medical Devices:
The purpose of a 510k submission is to provide the FDA with documented evidence proving that your medical device is substantially equivalent to a predicate device, which has already been approved for marketing by the FDA. Requirements for an FDA submission include A Documented Design Controls process, including intended use, indications for use, design inputs, design verification, and anlaytical validation studies. Clinical studies may be required for some, but not all 510(k) submissions.
A PMA is more in-depth than a 510k – it proves that a new device is safe and effective for the end user. It typically requires clinical trials with human participants and analytical validation studies. The standards here are much higher than for 510k submissions, and the FDA has just 180 days to accept or reject the application.
We have over 15 years of experience in custom assay development, we can confidently handle your project. Contact Us.
